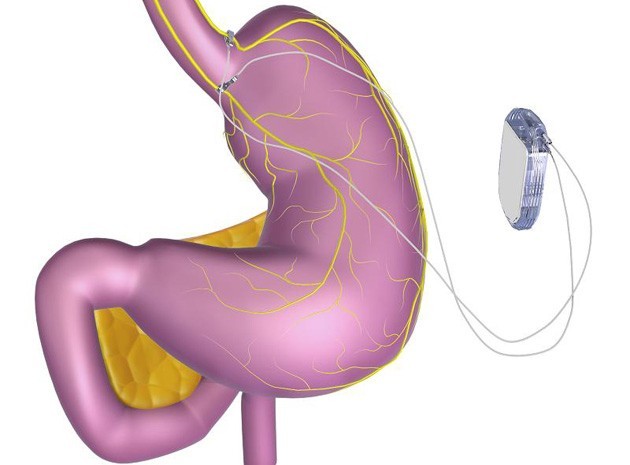
Before now, most heart pacemakers require some incision to get it up and running, that’s not the case for the Micra TPS pacemaker. Medtronic plc announced today that their Micra TPS pacemaker “world’s smallest pacemaker” just received U.S. Food and Drug Administration (FDA) approval. It’s a big news for the medicine world as this is the first device approved by the US government to employ the miniaturized pacing tech. The new pacemaker is a tenth of the size of traditional pacemakers and it’s small enough to be delivered through a catheter and implanted directly into the heart thereby eliminating the complication associated with cardiac wires (leads).
The Micra TPS pacemaker is about the size of a large vitamin. The tiny pacemaker attaches to the heart with small tines and delivers electrical impulses that pace the heart through an electrode at the end of the device. The tines and the share size of the Micra TPS pacemaker makes it cosmetically invisible as it eliminates the need for leads or a surgical “pocket” like in traditional devices. That’s not all the advantages of the Micra TPS pacemaker. The device responds to the patients’ activity levels by automatically adjusting therapy. It’s also approved for use in both 1.5 and 3 Tesla (T) full-body magnetic resonance imaging (MRI) scans, providing patients with access to the most advanced imaging diagnostic procedures available.
Even though the device is designed to be left in the body, the Micra TPS pacemaker design comes with a retrieval feature to enable retrieval when possible. The device is designed in such a way that it can be permanently turned off so it can remain in the body and a new device can be implanted without risk of electrical interaction. The company noted that almost all patients, 98.3 percent (292 of 297), had low and stable pacing thresholds at six months, yielding projected average longevity for the device of more than 12 years.